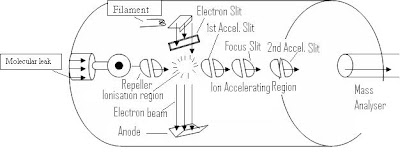
The electron impact ionization is highly developed and most commonly used ionization method in mass spectrometer. In Electron Impact ionization the chamber is maintained at a pressure of 0.005 torr.
The gaseous neutral sample molecules enter into the ion chamber through the molecular leak. A tight helical beam of highly energetic accelerated electron beam (70ev) from a glowing tungsten or Rhenium filament pass perpendicular to the incoming gas molecule.
These electrons are drawn off by a positive charged slit called as electron trap, which is on the opposite side of the filament. Thus electrons travel across the ion chamber. Ions are generated by the exchange of energy during the collision of the electron beam and sample molecules. As the electron beam is highly energetic (70 ev), it provides sufficient energy to gas molecules to have ionization and to cause the characteristic fragmentations of sample molecules either by loss of radicals or by loss of neutral molecules.
e- (70eV)
M ------------------> M+. + 2е-
M + .------------> A+ + B .
M + . ---------------> A+ . + B
A strong electrostatic field between the 1st and 2nd accelerating slits of 400-4000 V, accelerates the ions of various masses to their final velocities. The ions emerge from the final focusing slit
(0 volts) as a collimated ion beam with velocities and Kinetic Energies as
zV = ½ m1 υ 1 2 = ½ m2 υ 2 2 = ½ m3 υ 3 2 = ………….
Where
z = Charge of the ion
V = Voltage
υ = Velocity of various ions
m = Mass of respective ion
CLEAR PICTORIAL EXPLANATION
ReplyDelete