ORGANIC SPECTROSCOPY
Search This Blog
Sunday 15 April 2012
Sunday 11 March 2012
DETECTORS IN MASS SPECTROMETER: FARADAY CUP
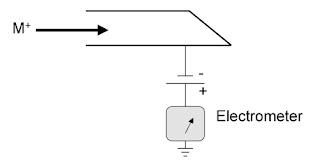
A Faraday cup is a metal cup designed to catch charged particles in vacuum. When a beam or packet of ions hits the metal it gains a small net charge while the ions are neutralized.
The metal can then be discharged to measure a small current equivalent to the number of impinging ions. By measuring the electrical current by electrometer in the metal part of the circuit, the number of charges being carried by the ions in the vacuum part of the circuit can be determined by using this expression.
The metal can then be discharged to measure a small current equivalent to the number of impinging ions. By measuring the electrical current by electrometer in the metal part of the circuit, the number of charges being carried by the ions in the vacuum part of the circuit can be determined by using this expression.
N/t = I/e
Where
N = Number of ions observedt = time taken in seconds
I = Measured Current (in amperes)
e = charge of electron (about 1.60 × 10-19 C).
Faraday cups are not as sensitive as electron multiplier detectors, but are highly regarded for accuracy because of the direct relation between the measured current and number of ions.
DETECTORS IN MASS SPECTROMETER: ELECTRON MULTIPLIER
An electron multiplier (continuous dynode electron multiplier) is a vacuum-tube that multiplies incident charges. They consist of a series of biased dynodes .( PbO coated surface) that eject secondary electrons when they are struck by an ion, in a process called secondary emission induce emission of roughly 1 to 3 electrons. If an electric potential is applied between this metal plate and another, the emitted electrons will accelerate to the next metal plate and induce secondary emission of still more electrons. This can be repeated a number of times, resulting in a large shower of electrons all collected by a metal anode, all having been triggered by just one.
The avalanche can be triggered by any charged particle hitting the starting electrode with sufficient energy to cause secondary emission. Hence the electron multiplier is often used as an ion detector. It could also be triggered by a photon causing vacuum photoemission of at least one electron. In a photomultiplier tube, a photo-emissive surface is followed by an electron multiplier with several sequential multiplying electrodes called dynodes. Because these electrodes are separate from each other, this might be called a "discrete-dynode" multiplier.
DETECTORS IN MASS SPECTROMETER: DALY DETECTOR
Daly Detector:
A Daly detector is a gas phase ion detector that consists of a metal "doorknob", a scintillator (phosphor screen) and a photomultiplier. Ions that hit the doorknob release secondary electrons. A high voltage (ca. -20,000 V) between the doorknob and the scintillator accelerates the electrons onto the phosphor screen where they are converted to photons. These photons are detected by the photomultiplier.
Advantages:
As acceleration is done , it can improve the sensitivity for high mass ions in case of Time of Flight analyzer.
Friday 22 July 2011
NMR THEORY-Orientation of a nuclei in presence of Magnetic Field
The nuclei of many elemental isotopes possess an Intrinsic spin, therefore they are associated with an angular momentum μ . The total angular momentum of a nucleus is given by
μ = h/2π . [I(I+1)]
Where
h= Planks Constant
I= Nuclear Spin or Spin Number having values as 0, 1/2, 1, 3/2.......
Note: If I = 0, then the nucleus does not posses a spin.
The numerical values of the spin number I is related to the mass number and the atomic number as follows
Atomic Number | Mass number | No. of Protons | No. of Neutrons | Spin Quantum no. | Examples |
even | even | even | even | I=0 | 12C,16O |
even | odd | even | odd | I= ½,3/2 , 5/2 | 13C |
odd | odd | odd | Zero or even | I= ½,3/2 , 5/2 | 1H |
odd | even | I = 1,2,… | 14N,2H |
Since atomic nuclei are also associated with an electric charge, the spin give rise to a magnetic field. Therefore a spinning nucleus may be considered as a minute bar magnet.
In absence of magnetic field the nucleus is oriented in random orientations and they have a degenerate energy level that is equal energies.
In a magnetic field the angular momentum of a nucleus (I>0) is quantized, the nucleus takes up one of (2I+1) orientations with respect to the direction of the applied field.
Each orientations corresponds to a characteristic potential energy of the nucleus equal to
E = μ . H0 . Cos θ
Where
Ho = Strength of applied field
θ = Angle which the spin axis of the nucleus makes with the direction of the applied field.
Wednesday 20 July 2011
Shielding and Deshielding- Chemical Shift
The changes in absorption frequency due to shielding is called the chemical. Electrons, like nuclei, have an inherent magnetic field(both have spin=1/2). But the strength of the magnetic field of electron is several thousands of times stronger than a nuclei. As the electrons charge is negative, the polarity of the magnetic field generated by an electron is opposite that of the nucleus i.e., in an external magnetic field, the lowest energy nuclei "align with" the external field where as the lowest energy electrons "align against" the external magnetic field.
In a molecule, the nucleus is always surrounded by an electron cloud. as a result the nucleus will experience an effective magnetic field (Heff).
Note: Heff is a combination of the external applied magnetic field and the magnetic field generated by the electron cloud surrounding the nucleus.
With in a molecule there are factors which can increase or decrease the electron density surrounding a nucleus. Factors which reduce the electron density are said to deshield the nucleus(Heff at the nucleus increases. Similarly, factors which increase the electron density are said to shield the nucleus since Heff will decrease.
Heff = Ho + σ
Subscribe to:
Posts (Atom)